Our Test at Glance
We are the first company to produce a mast cell line that remains both stable and functional outside the body. Combined with our know-how and advanced data analytics, FAST-PASE® directly replicates the allergic response — delivering results that are reliable, reproducible, and safe.
Inside an allergic reaction
During an allergic reaction, a mast cell meets an allergen it is primed to recognize. The cell bursts into action — releasing histamine and other chemical signals that call the body’s defenses into play. This chain reaction is what produces the symptoms of allergy, from hives and swelling to, in severe cases, anaphylaxis.
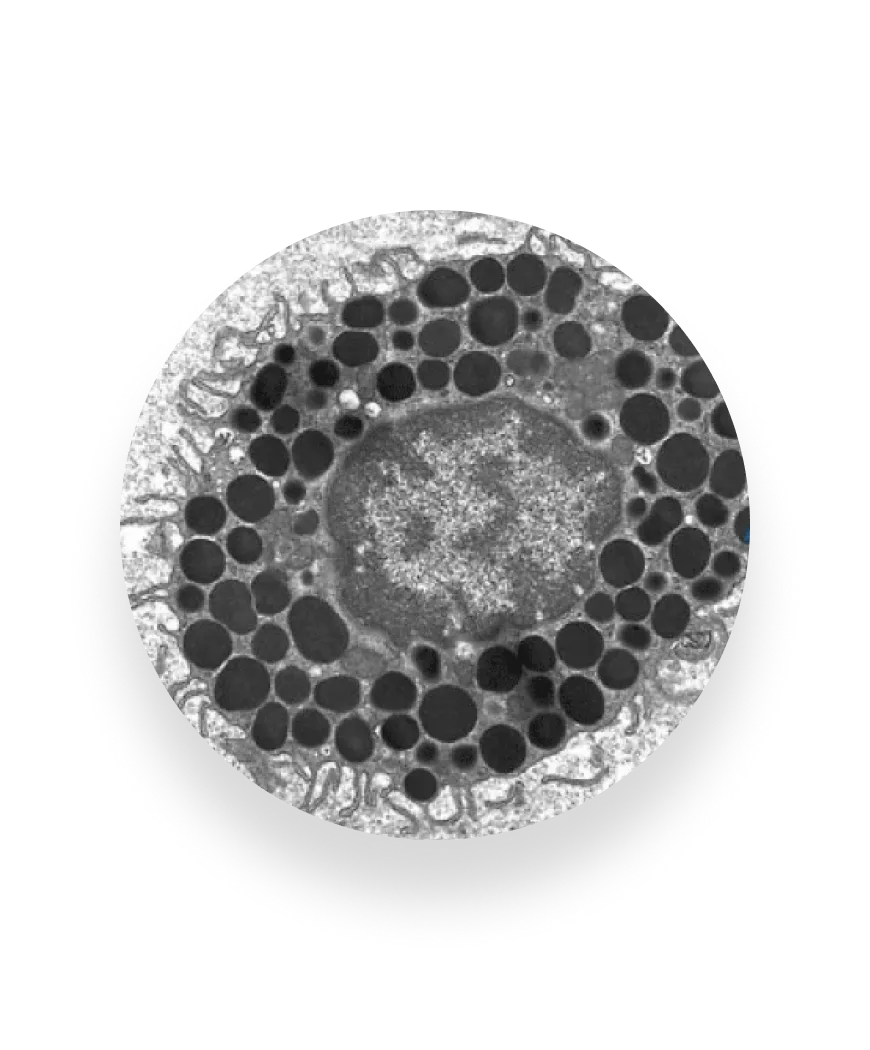
The immune response, recreated
Until now, the allergic reaction has been very hard to observe outside of patient’s body — primary cells lose function quickly, and immortalized lines often fail to behave like real allergy cells.
FAST-PASE® is powered by the first stable, functional mast cells that can live outside the body. By exposing these cells to a patient’s blood and defined allergens, we capture the body’s true immune response and deliver clear, reliable answers without putting patients in harm’s way.
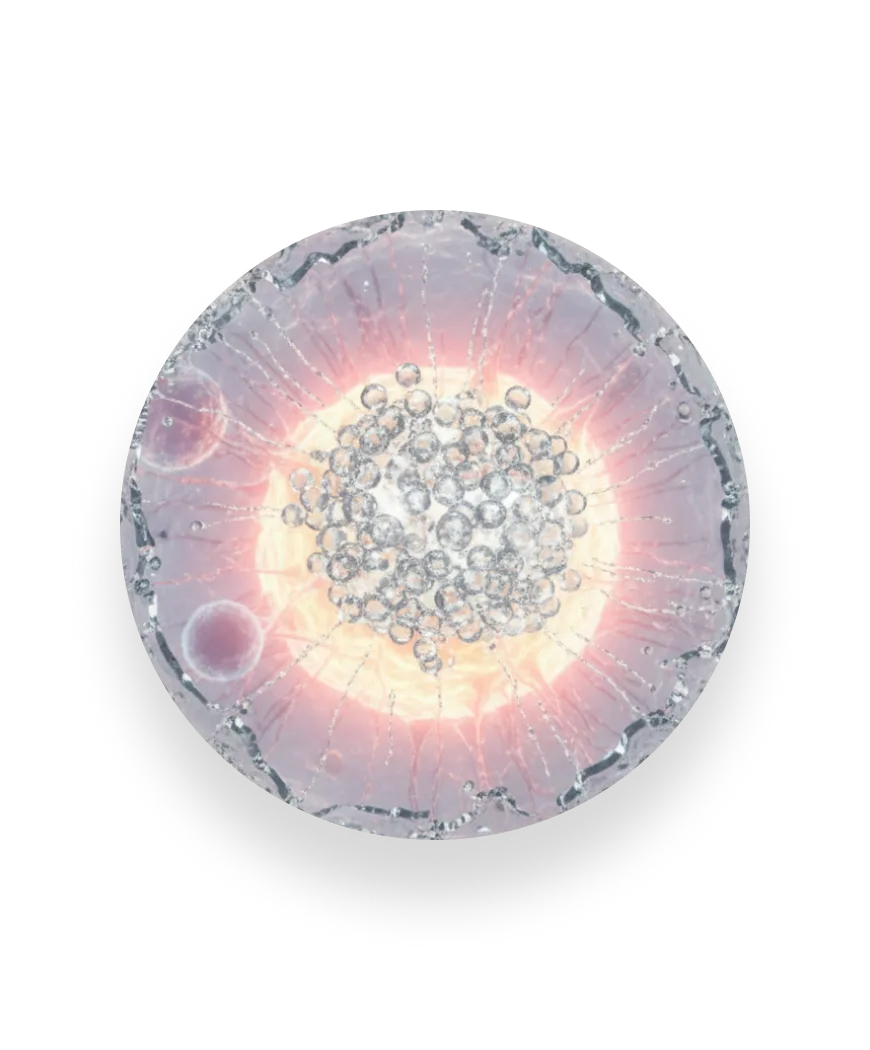
Measuring the full response
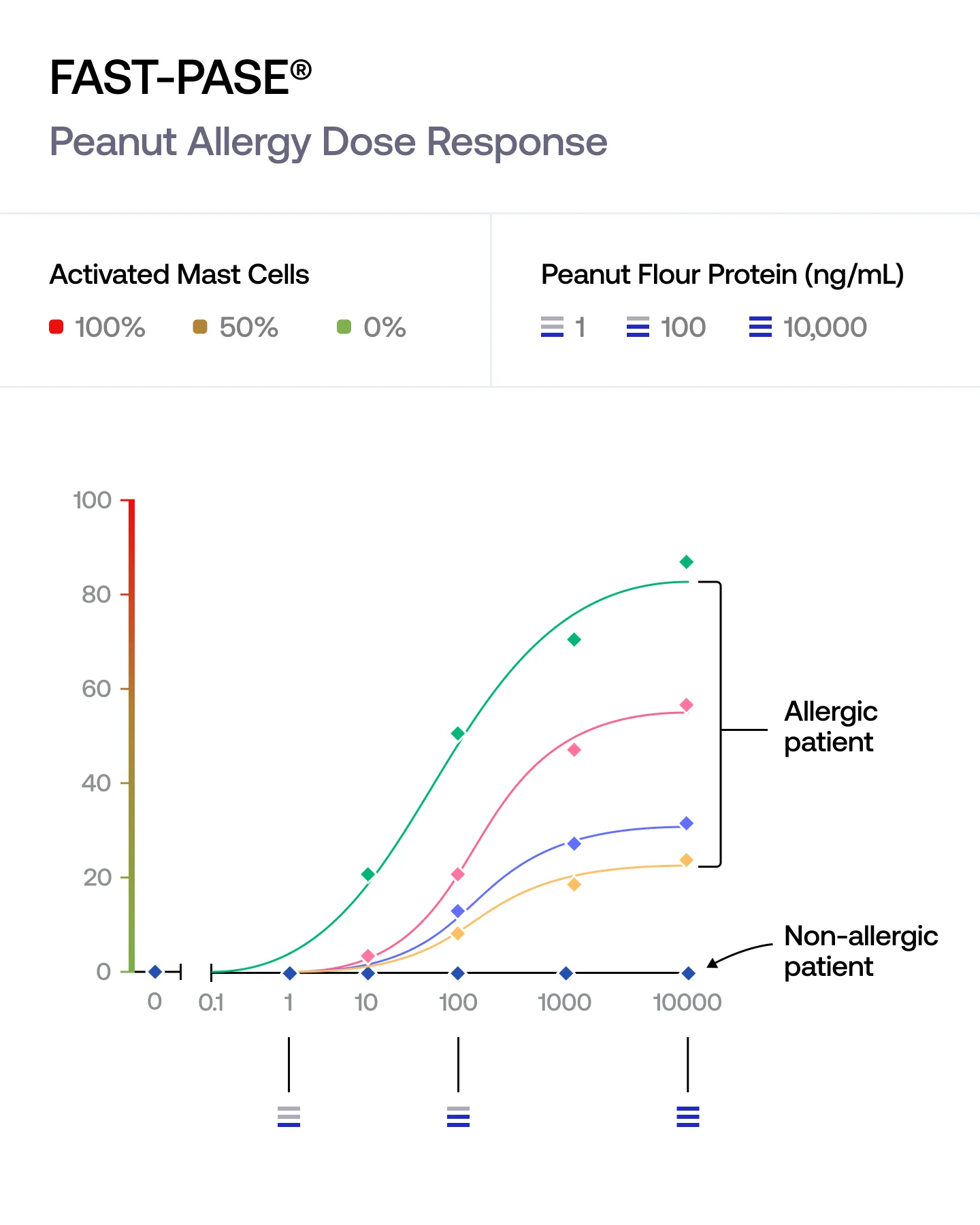
FAST-PASE® exposes our mast cells to a range of allergen concentrations and tracks how the response builds.
We capture the concentration at which the reaction begins, the strength of the response at each step, and the maximum activation reached. This reveals the full biology of an allergy — not just whether it happens, but how it unfolds.

Real-World Validation
FAST-PASE® has been used in allergy research on more than 10,000 patient samples* over the past decade and helped bring major allergy drugs to market.
Across these studies, FAST-PASE® has consistently aligned with what clinicians observe in practice reliably distinguishing true allergies from false positives.
By directly measuring cell activation, the test delivers results that are both specific and reproducible, giving providers the confidence that legacy methods often lack.

Scientifically proven
FAST-PASE® is validated in peer-reviewed publications and trusted by world-class scientific institutions and leading therapeutics companies.
The Future of FAST-PASE®
The next generation of FAST-PASE® will provide semi-quantitative readouts distinguishing strong, mild, and absent responses - enabling physicians to track treatment progress longitudinally during immunotherapy or anti-allergy drug therapy.
Collaborative Research
FAST-PASE® has become a trusted tool in research and development. The assay has already been used in large-scale efforts such as CoFAR (Consortium of Food Allergy Research) and in clinical programs run by leading pharmaceutical partners. Feedback from groups including the NIH and Novartis has been consistent: the data are clean, reproducible, and easy to interpret.

Scientific Leadership
Our multidisciplinary team includes immunologists, biophysicists, and optical engineers with extensive publication records and clinical trial experience. This fusion of expertise underpins Atanis’s scientific rigor and commitment to translating breakthrough science into diagnostic impact.






